The Crystal Structure of Titanium
Generally, pure titanium can crystallize in two crystal structures: α titanium and β titaniu. When it crystallizes at low temperatures (room temperature), the hexagonal close-packed (HCP) structure of alpha titanium is formed. While it crystallizes at high temperatures, the body-centered cubic (BCC) structure of beta titanium is formed. The complete transformation from one to another crystal structure is called allotropic transformation. The repsective transformation temperature is called the transus temperature. The HCP α titanium is stable at room/low temperatures, while the BCC β titanium is stable at high temperatures. The β transus temperature for pure titanium is 882±2°C.
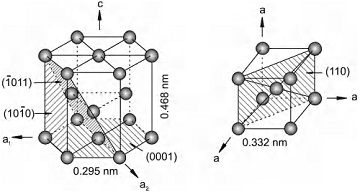
The crystal structure of α titanium of HCP and β titanium of BCC.
For unalloyed titanium, which is also known as commercially pure titanium, ASTM Grade 1, 2, 3, & 4, depending on the level of impurities, the β transus temperatures are usually higher than that of pure titanium. The microstructure of unalloyed titanium at room temperature is typically a 100% alpha-crystal structure. As amounts of impurity elements increase (primarily iron), small but increasing amounts of beta phase are observed metallographically, usually at alpha grain boundaries. Annealed unalloyed titanium may have an equiaxed or acicular alpha microstructure.
As schematically shown in above picture:
- For α titanium, the lattice constants: a = 0.295 nm, c = 0.468 nm; the close-packed direction: <1120>; the close-packed plane: {0001}; coordination number: 12; packing factor: 0.74; atoms per unit cell: 6.
- For β titanium, the lattice constants: a = 0.332 nm; the close-packed direction <111>; the close-packed plane: {110}; coordination number: 8; packing factor: 0.68; atoms per unit cell: 2.
